In most industries, a hiring mistake is expensive. In the pharmaceutical industry, it can shut down a line, trigger a warning letter, or delay a life-saving therapy.
That difference is the starting point for any serious conversation about pharmaceutical staffing.
Leaders in this space already understand recruitment mechanics. What they wrestle with is something deeper. How do you secure capability, prove competence, and maintain inspection readiness at the same time, especially when demand spikes, pipelines shift, or new facilities come online?
Why is pharmaceutical staffing fundamentally different?
Think of hiring in pharma like replacing parts in a flying aircraft. The machine never stops, production targets hold, documentation continues, and audits can land without notice.
Every person added to that system must fit immediately.
This is why pharmaceutical recruitment carries heavier expectations than most talent programs. You are protecting validated environments, data integrity, and patient safety.
In practical terms, hiring decisions intersect with:
- training matrices
- SOP adherence
- electronic systems access
- deviation exposure
- inspection traceability
A resume is only the surface, whereas the real evaluation sits behind it.
Where pressure is building for Pharma HR leaders
Enterprise organizations feel it first, but mid-sized manufacturers catch up quickly once expansion begins.
New product introductions, tech transfers, remediation projects, and serialization initiatives multiply the demand for people who can operate within good manufacturing practice expectations from day one.
Traditional pipelines struggle here. The talent exists, yet availability, geography, and documentation rarely align when you need them.
That gap is where modern pharmaceutical talent acquisition strategies are evolving. Speed matters here, but defensibility matters more.
Staffing models used in pharma
The conversation has matured far beyond temporary labor. Today’s pharma industry hiring strategy blends multiple engagement structures depending on validation risk, knowledge continuity, and time horizon.
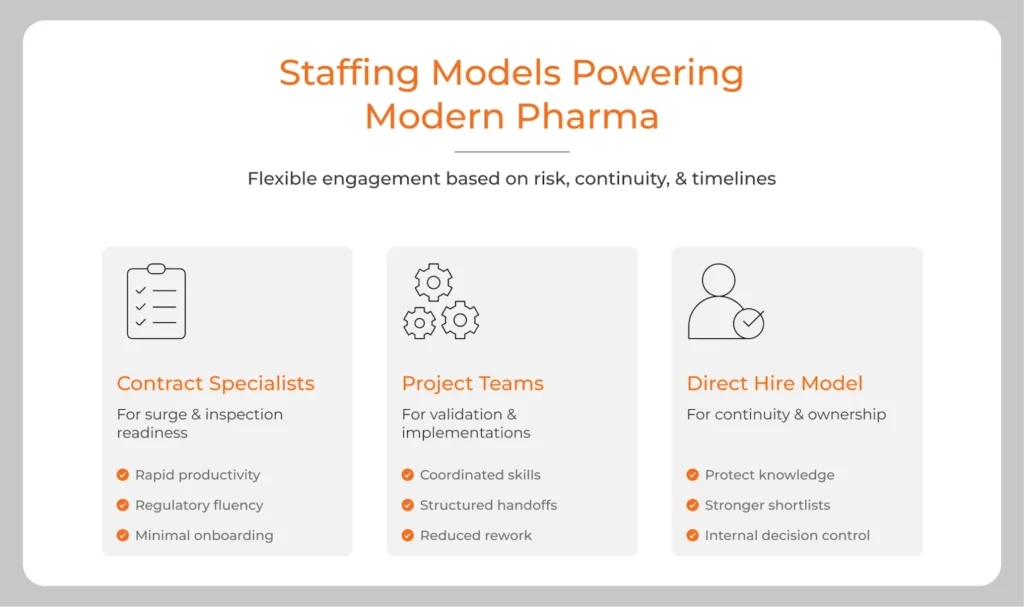
Contract specialists for surge activity
During audits or product scale-ups, organizations bring in experienced professionals who have lived through inspections. They understand batch records, change control, CAPA rhythms.
They contribute quickly because they recognize the language of the environment.
Project-based teams
For validation, commissioning, or digital implementations, companies often secure pods of talent through project based staffing solutions providers who can deploy coordinated, interdependent skill sets rather than isolated individuals.
Consider a system go-live. CSV engineers execute protocols. QA reviewers ensure alignment with quality expectations. Document control specialists maintain version integrity and traceability. Each role depends on the other. Delays in one area quickly cascade into the rest.
Teams that have worked within regulated programs understand handoffs, escalation paths, and approval mechanics. They arrive with a shared rhythm. Communication tightens, rework declines, and internal subject matter experts spend less time orchestrating movement and more time validating outcomes.
The real value of project staffing lies in integration speed and collective reliability.
Direct hires supported by partners
Certain positions carry long-term ownership expectations. Process knowledge, site history, and cultural alignment make permanent employment essential for continuity. In these situations, organizations may still rely on external expertise to strengthen the front end of the funnel.
Partners contribute structured sourcing, specialized screening, and rigorous credential validation. They understand how to probe for regulated experience, training depth, and evidence of operating inside controlled environments. By the time candidates reach hiring managers, baseline risk has already been reduced.
Internal leaders remain firmly in control of selection, assimilation, and performance development. The final decision sits where accountability resides.
The direct hire model allows companies to protect institutional memory while accelerating access to hard-to-find talent.
And like every effective pharma hiring approach, it works best when used deliberately. High-performing organizations blend permanent hiring and external support based on risk profile, urgency, and the level of knowledge that must stay embedded within the business.
How staffing partners support pharma compliance
Here is where experienced buyers sharpen their focus. Submitting candidates is easy, but only a few can defend them during an inspection.
Effective pharma staffing partners build mechanisms upstream of deployment.
They verify employment histories with attention to regulated exposure. They map competencies against GMP expectations. They confirm training evidence and document everything.
When done right, this preparation reduces friction in three areas.
First, onboarding accelerates because documentation is ready. Second, supervisors spend less time remediating skill gaps. Third, audit narratives remain clean when regulators ask how personnel qualification was assured.
The hiring is faster and audits queiter.
What roles organizations commonly externalize?
Outsourcing decisions in regulated environments are rarely driven by convenience. They are shaped by two forces that every pharma leader recognizes immediately. Risk tolerance and labor volatility.
When inspection exposure is high or timelines are compressed, organizations expand capacity in places where specialized expertise can be introduced without weakening control. The sponsor retains ownership. The partner supplies momentum.
Here is how that typically plays out across functions.
Quality assurance operations
QA outsourcing usually focuses on repeatable but essential activities that can slow down internal teams during peak periods.
Think batch record review backlogs after a production surge, deviation closures piling up before an inspection window, or document updates after procedural changes.
Experienced external QA professionals step in with muscle memory already built. They understand review discipline, data integrity expectations, and how to write defensible rationales.
Internal leadership still approves decisions, release authority does not move, but what changes is throughput.
Quality control laboratories
Labs experience some of the sharpest volatility in the enterprise.
New product introductions, stability commitments, method transfers, and investigations can swing the workload dramatically within weeks. Hiring permanently for those spikes leaves excess capacity later.
Specialist contractors provide relief without distorting long-term headcount models. They arrive already familiar with instrument qualification norms, data recording practices, and lab safety culture.
Supervision, final approvals, and trending remain internal while sample queues start moving again.
Validation and engineering
Few disciplines are as project-driven as validation.
A new line installation or digital system rollout may require dozens of specialists for a defined window, but after go-live, demand drops sharply.
Partners supply commissioning teams, protocol authors, and execution engineers who have delivered similar programs elsewhere. They know the documentation rhythm and anticipate typical findings.
Your internal experts remain accountable for strategy and final acceptance. External talent accelerates completion.
Regulatory affairs documentation
Submission cycles create intense documentation pressure. Whether preparing variations, renewals, or market expansions, regulatory groups often need temporary depth in publishing, formatting, or technical writing.
Here, outsourcing protects deadlines. Contributors familiar with authority expectations can align documents quickly while internal SMEs validate scientific and strategic accuracy.
Ownership of the filing never leaves the sponsor, and timelines become manageable.
Manufacturing support during expansions
Scale-ups present a paradox. You must increase output immediately while preserving culture, compliance, and efficiency.
Temporary operators, supervisors, and technical specialists provide breathing room. They help stabilize shifts, maintain yield, and protect schedules while permanent hiring progresses at a responsible pace.
Leadership retains process control and performance management, and capacity flexes without surrendering governance.
Also Read: Manufacturing Workforce Solutions: How to Build a Skilled, Scalable Workforce
Across all these areas, the principle is consistent. Accountability stays with the sponsor; decision rights remain internal. What changes is execution bandwidth.
And that distinction matters enormously during pharmaceutical hiring cycles linked to approvals, remediation programs, or facility upgrades.
Demand arrives in waves. Flexible talent models allow organizations to meet those waves without compromising validated systems or exhausting core teams.
The companies that handle this well add capability exactly where the risk curve is steepest, then scale it back with equal precision once stability returns.
To Conclude
Hourly rates are easier to benchmark than regulatory exposure. Delayed releases, repeat deviations, and fragile documentation trails often outweigh any savings achieved in procurement. This is why experienced leaders approach pharmaceutical staffing as a question of continuity, noticing that professionals who arrive inspection-ready tend to protect far more value than they cost.
The market is now demanding proof of that readiness across blended workforces. Organizations are moving toward real-time visibility of qualifications, training currency, and deployment risk. As expectations rise, the partners who stand apart are those who can deliver talent and defend it under scrutiny.
If strengthening compliance confidence while maintaining operational momentum is on your agenda, SPECTRAFORCE can help you build a workforce model designed for both. Explore our staffing solutions today.
FAQs
Pharmaceutical staffing solutions are structured services that provide qualified professionals with documented, regulated experience, validated competencies, and readiness to operate within GMP environments.
Staffing firms ensure GMP compliance by verifying employment histories, mapping skills to regulated tasks, confirming training evidence, and maintaining documentation that supports inspection defensibility.
Roles commonly outsourced in pharma staffing include quality assurance, quality control, validation engineering, regulatory affairs support, and manufacturing surge requirements.
Contract staffing can work in regulated pharma environments when qualification processes, oversight mechanisms, and documentation standards align with internal quality systems.
Pharma staffing reduces audit risk by supplying personnel whose competencies are pre-validated, documented, and traceable, which strengthens narratives around training and qualification during inspections.



